Gamp Good Practice Guide: GXP Compliant Laboratory Computerized Systems (2 Edition) | PDF | Verification And Validation | Systems Theory

Amazon.fr - GAMP 5: A Risk-based Approach to Compliant Gxp Computerized Systems - Ispe Headquarters - Livres
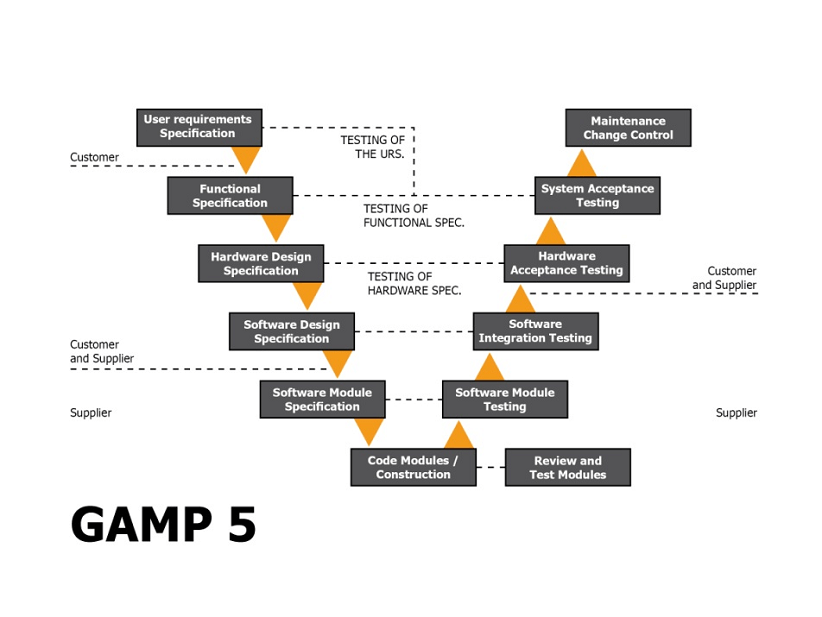
GAMP 5® – Innovation in a Flexible Manner - LearnGxP: Accredited Online Life Science Training Courses